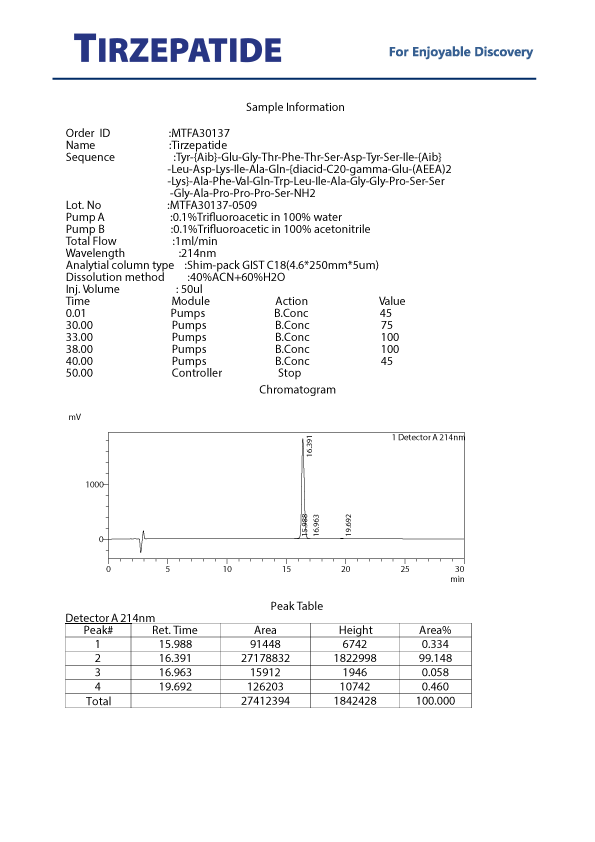
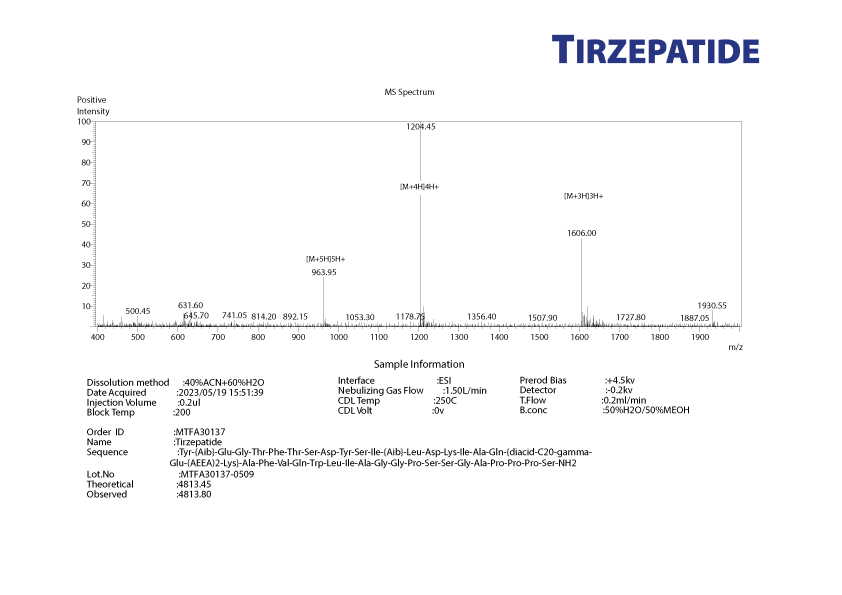
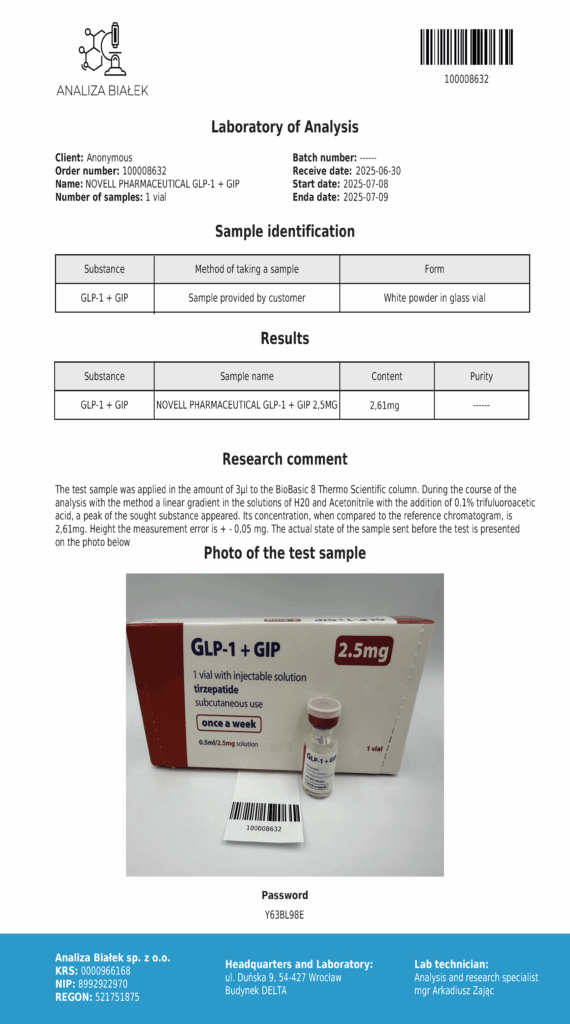
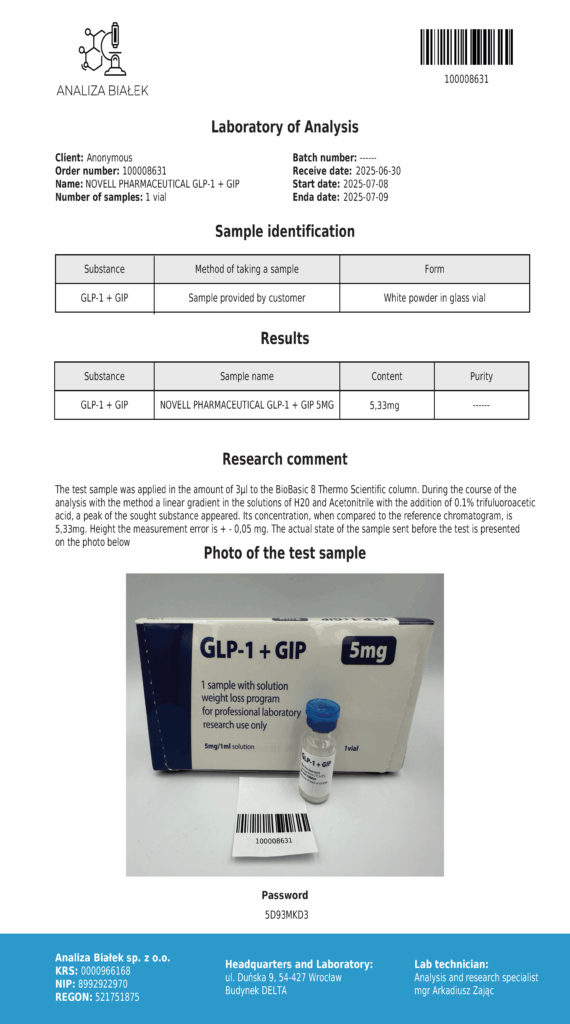
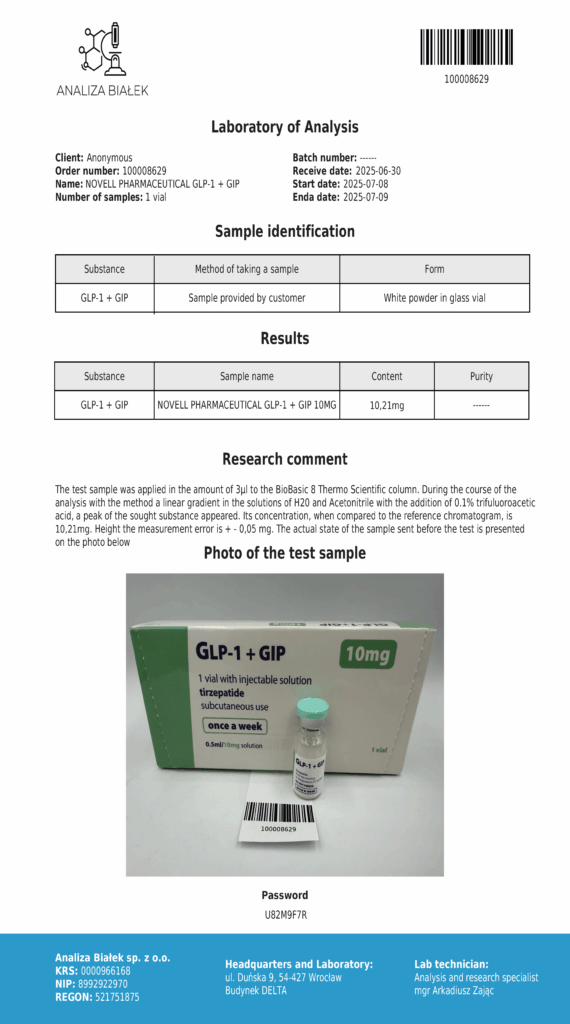
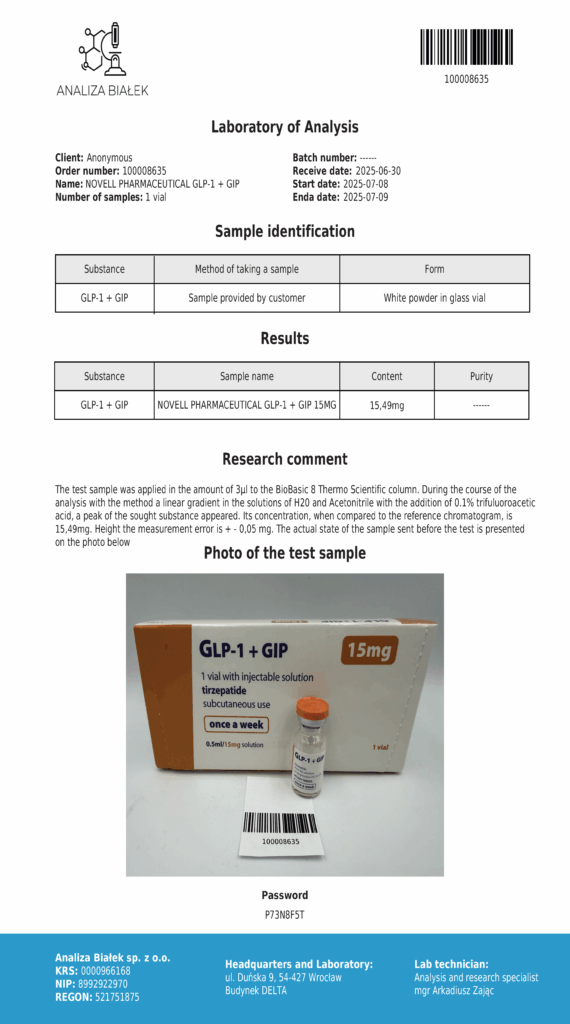
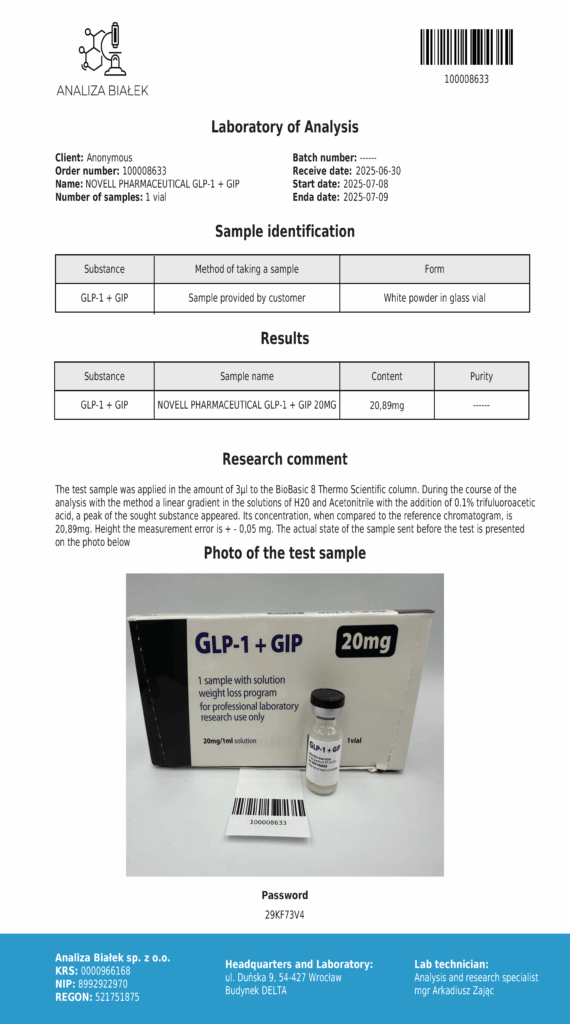
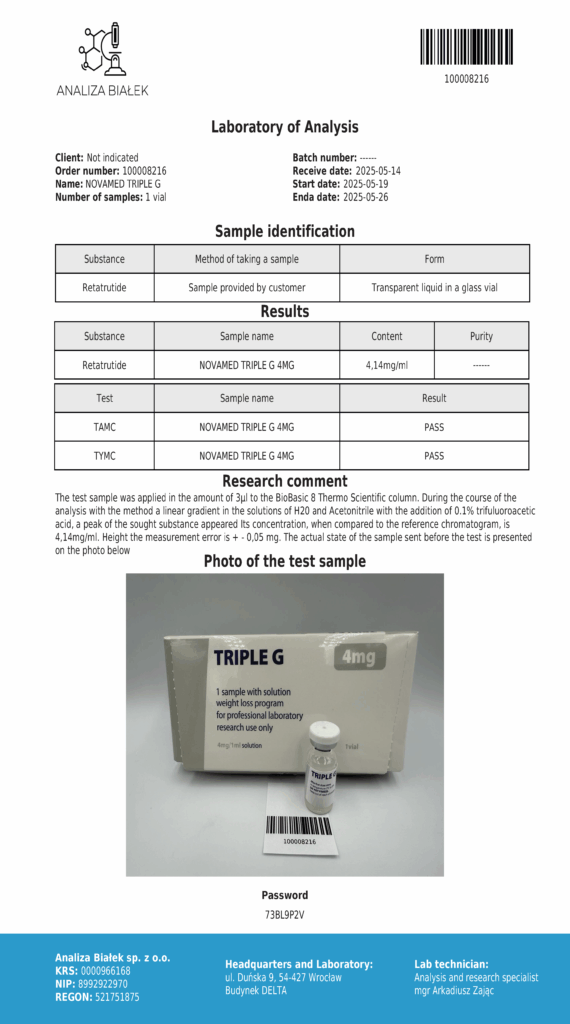
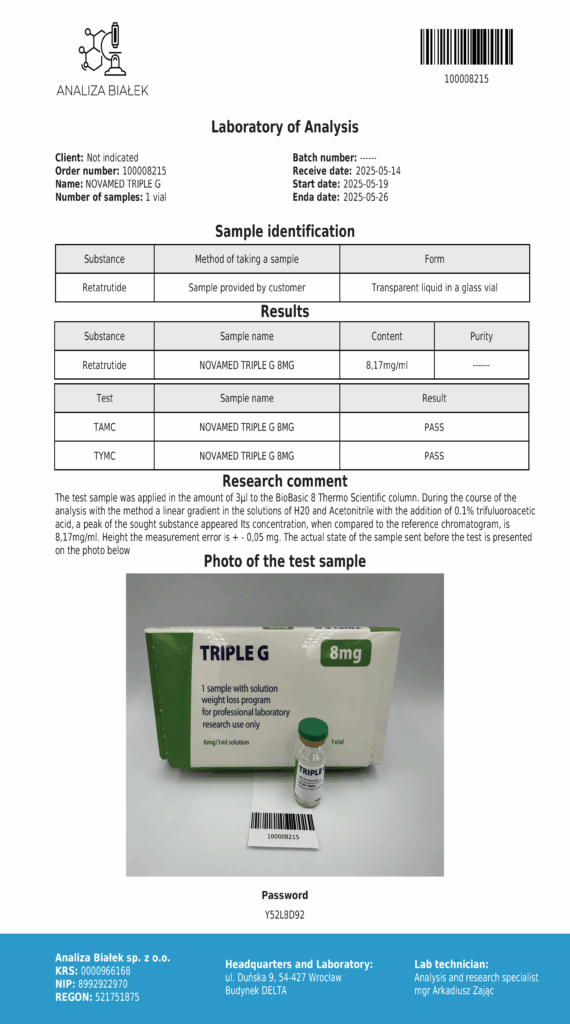
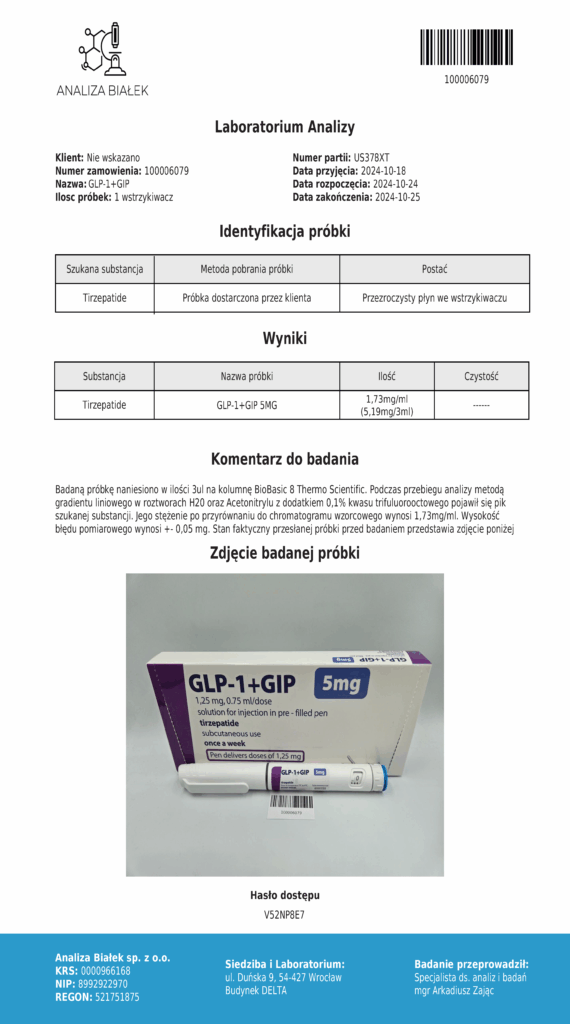
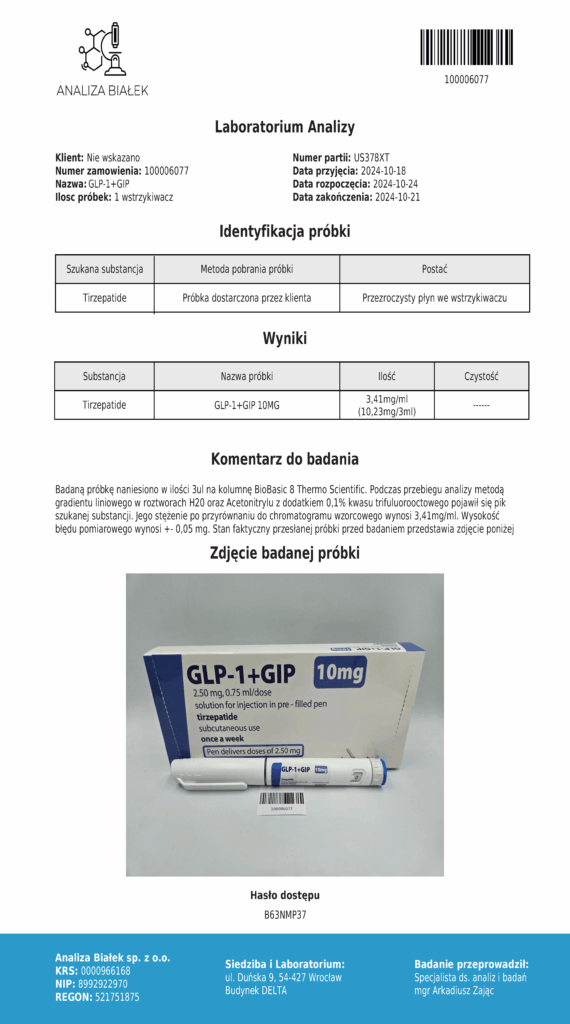
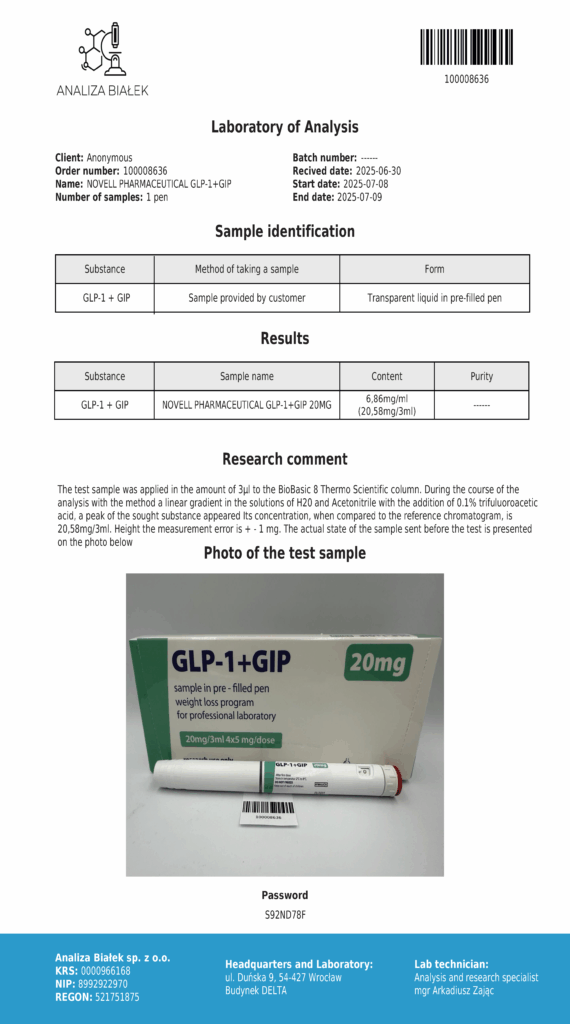
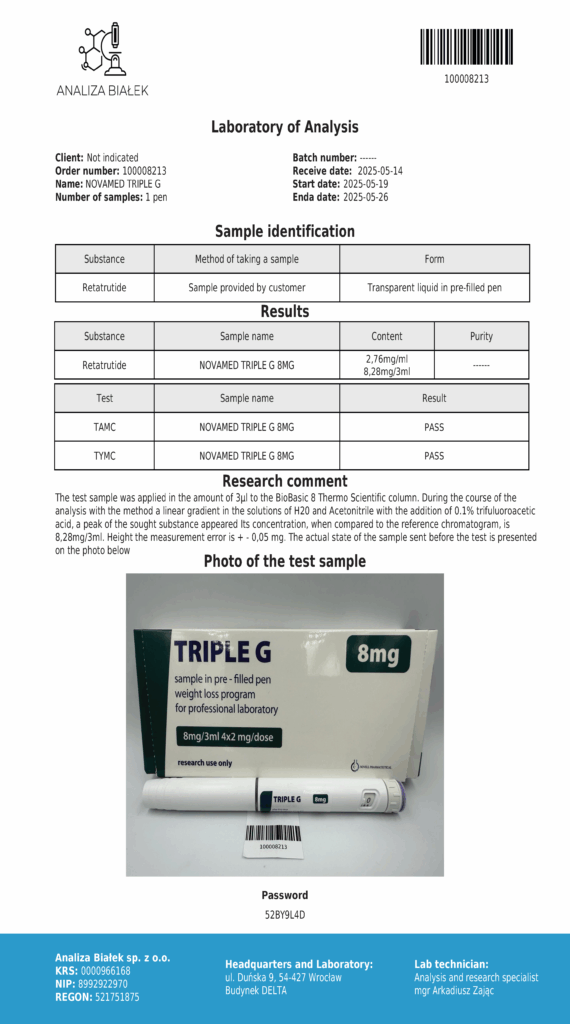
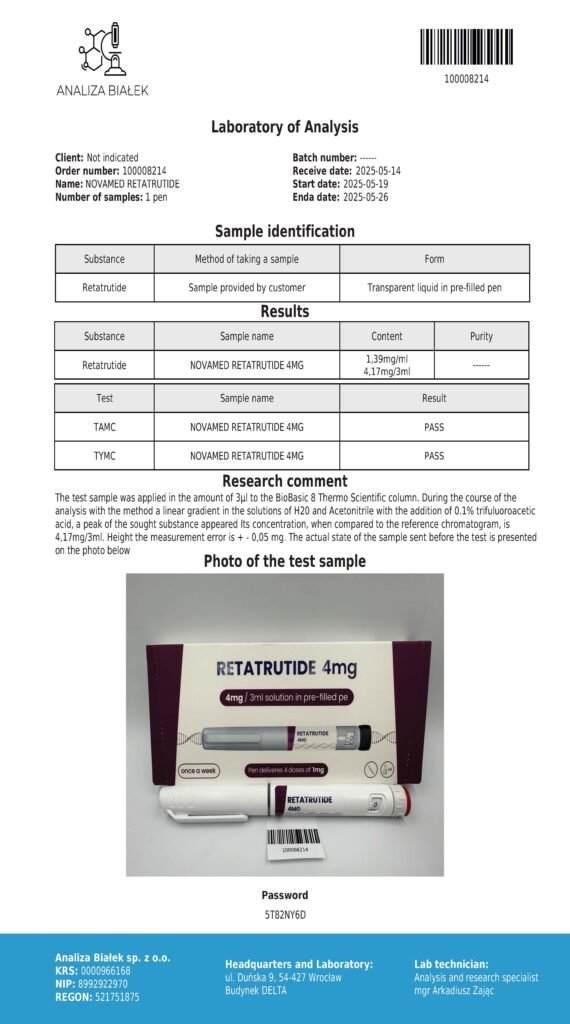
Laboratory testing and analysis to confirm quality and authenticity of products
Tirzepatide studies using the peptide
Clinical trials are a key step in evaluating the efficacy and safety of new drugs. Research conducted on Tirzepatide included different groups of patients, those who were healthy and overweight, as well as those with type 2 diabetes. Importantly, these studies yielded important data on Tirzepatide's effectiveness in lowering blood sugar and the drug's effect on other health indicators.
The results of the Tirzepatide study are promising. The drug has been shown to significantly lower blood glucose levels in study patients . In addition, Tirzepatide contributes to weight reduction, which is important for people who are overweight or obese. Interestingly, those using the product tended to experience significant weight loss without significant dietary changes. Studies have also shown favorable effects of Tirzepatide on indicators related to lipid profiles and kidney function. This means that the drug itself is not only safe, but also brings many potential health-promoting benefits.
GLP-1, GIP, GLUKAGON analogues and observation in medicine surpass study
Tirzepatide is an innovative agent that helps treat obesity. Here is some important information to remember:
First, the effectiveness in lowering blood sugar levels. Tirzepatide is an effective drug in lowering blood glucose levels in patients with type 2 diabetes, and clinical trials have confirmed its effectiveness in controlling blood sugar and improving patient outcomes.
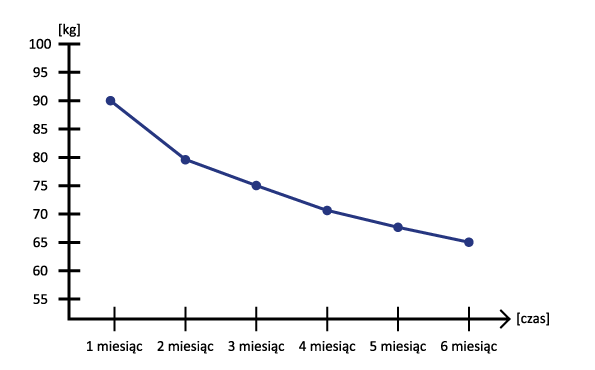
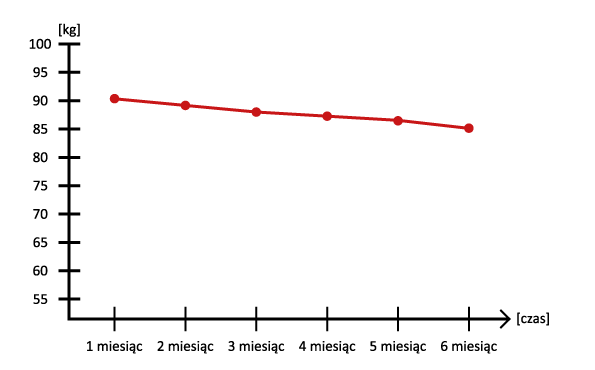
Secondly, a very large reduction in body weight and body fat without additional physical activity. Of course, physical activity promotes the effects of administration, but studies have confirmed that it is not necessary. We, however, recommend combining Tirzepatide with cyclic physical activity and a varied diet, or a caloric deficit ( in the case of very high overweight ).
Thirzepatide can contribute to weight loss. This is particularly important for people who are overweight or obese, as weight loss can improve blood sugar control. Importantly, the weight loss took place without any apparent dietary interference, making it much easier to reach the target weight.
And third and most importantly, the safety of use. Tirzepatide is generally well tolerated by patients. Like most drugs, it can cause some side effects, such as nausea, vomiting or diarrhea. However, most people tolerate the drug well, which further supports its use.
Lifestyle change. Tirzepatide is not a replacement for a healthy lifestyle, and thus patients should continue to maintain a proper diet, regular physical activity and control stress levels. Tirzepatide only provides support for the treatment of diabetes. The better you fine-tune your nutrition and incorporate physical activity, the better results you will get.
In conclusion, Tirzepatide is an effective product for the treatment of overweight and obesity. The results obtained with the peptide depend on the individual case and are influenced by lifestyle changes, among other things.
1. SURPASS TEST
Research in the series SURPASS (Surpass 1, 2, 3, 4) form the basis for the approval of thiothrombid for the treatment of type 2 diabetes. These Phase I, II and III clinical trials were conducted on large groups of patients and were designed to evaluate the efficacy of thiothrombid compared to other drugs used to treat diabetes (such as semaglutide, insulin therapy or metformin).
Main findings:
- Glucose control: Thioparatide significantly lowered glycated hemoglobin (HbA1c) levels, indicating improved blood sugar control.
- Weight reduction: Patients who used Tirzepatide experienced significant weight loss compared to the control group.
- Safety: Tirzepatide has shown an acceptable safety profile. The most commonly reported side effects are gastrointestinal problems such as nausea, which generally resolve after several weeks of therapy.
2. SURPASS-2 STUDY
As part of the study SURPASS-2 compared the efficacy of Tirzepatide with semaglutide, which is also a drug used to treat type 2 diabetes. The results showed that Tirzepatide was superior to semaglutide in terms of both HbA1c reduction and weight reduction. In addition, Tirzepatide was well tolerated, and side effects were similar to those observed in other studies.
3. SURPASS-3 STUDY
W SURPASS-3 evaluated the use of Tirzepatide in patients with type 2 diabetes who were not achieving sufficient glucose control with insulin. Thiothiopatide proved more effective in lowering HbA1c levels and reducing body weight than basal insulin.
4. SURPASS-4 STUDY
Study SURPASS-4 focused on patients who did not respond to metformin treatment. Thiothiopatide proved more effective in reducing HbA1c compared to placebo and showed a beneficial effect on body weight.
Summary of results:
- Thiothiazepatide versus glucose control: It definitely improves blood glucose control, both in monotherapy and in combination with other drugs.
- Thioparatide versus weight loss: Its use is associated with marked weight loss, which is an added benefit, especially in overweight and obese patients.
- Safety: Thiothiopatide shows a good safety profile, although some patients may experience side effects such as nausea or diarrhea, which usually resolve after a short time.
Conclusions:
Thioparatide is a very promising drug that offers patients with type 2 diabetes an effective alternative for blood sugar control and weight reduction. Its effectiveness has been confirmed in a number of clinical trials, making it a valuable tool in the treatment of this disease.
Sources:
https://pubmed.ncbi.nlm.nih.gov/35658024/
https://pubmed.ncbi.nlm.nih.gov/34170647/
https://pubmed.ncbi.nlm.nih.gov/33325008/
All the properties mentioned above were observed with laboratory tests. They are for informational purposes only. Any information contained in the descriptions is not approved by GIS, GIF or EFSA. The substance is not a drug, food product or dietary supplement. It is not suitable for human consumption. The product is qualified as a chemical reagent / reference material approved for marketing in the EU. It can only be used for scientific research. Other information about the agent is contained in the Material Safety Data Sheet, which is available for review. Products are available to institutions or individuals who are associated with research or laboratory activities.